Ionic Bond Formation/ Ionic Compound Formation
Ionic bond Formation:
Sodium ion (Na+)Formation:
In ionic bond formation cation ( formed from metal atom) which is formed by lose of electron/s and gets positive charge gets attached to anion (formed from non-metal) by electrostatic force of attraction. Anion is formed by accepting that/those electron/s donating by cation. in case of ionic bond there is no bond by electrons rather opposite charged ions are attached just due to opposite charges.
Here we will understand the ionic bond formation by sodium chloride (NaCl) .Sodium ion (Na+)Formation:
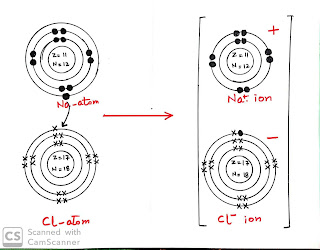
Sodium(Na) is an atom being atom it is neutral. Its atomic number is 11 means it contains 11 protons in nucleus and 11 electrons in shells. Its valence shell (outer most shell which determine valency) has one electron as shown in the figure. to get stable by octet rule it loses its one electron from its valence shell and get configuration of nearest nobel gas i.e neon(Ne) as shown in figure
Chlorine (Cl ) is an atom and neutral . Its atomic number is 17, its mean it contains 17 protons in its nucleus and 17 electrons in its shells.Its valence shell has seven electrons as shown in figure , to get stable by octet rule it gains one electron and gets configuration of nearest nobel gas i.e argon(Ar)as shown in figure
Note above figures are "dot and cross" structure of atom. In dot and cross structure of atom electrons of one atom is represented by dot and electrons of other atom would be in cross.In compound anion would have one or more electrons of different which shows that these electrons belongs to cation.
 |
Chloride Ion (Cl-)Formation |
Ionic compound formation Sodium chloride(NaCl)
Sodium (Na), a metal atom, gives electron from outer most shell and chlorine (CL), a non metal atom, accepts that electron. They become Na+ cation and Cl- anion .These ions start to live together by electrostatic force of atraction and forms an ionic compound sodium chloride(NaCl) as shown below.
 |
| Sodium chloride NaCl formation (an ionic compound) |



✌
ReplyDelete